-40%
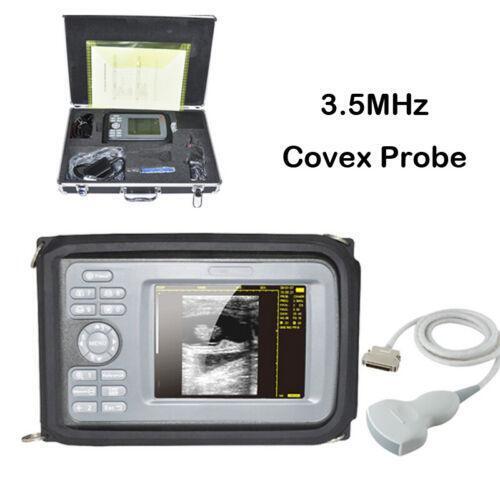
Portable Carejoy Convex Probe Ultrasound scanner LCD Hospital Handhold Human
$ 475.19
- Description
- Size Guide
Description
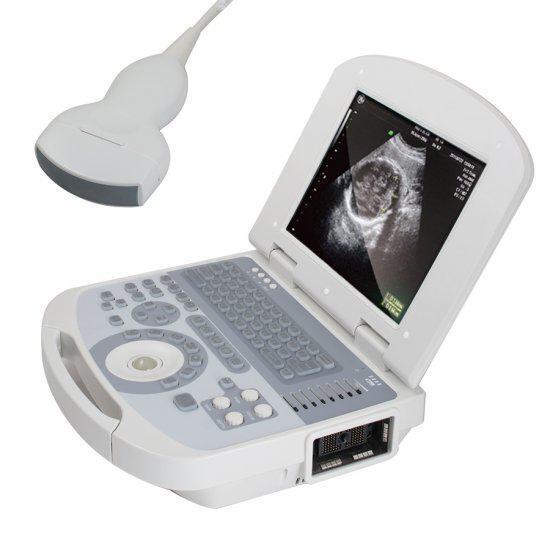
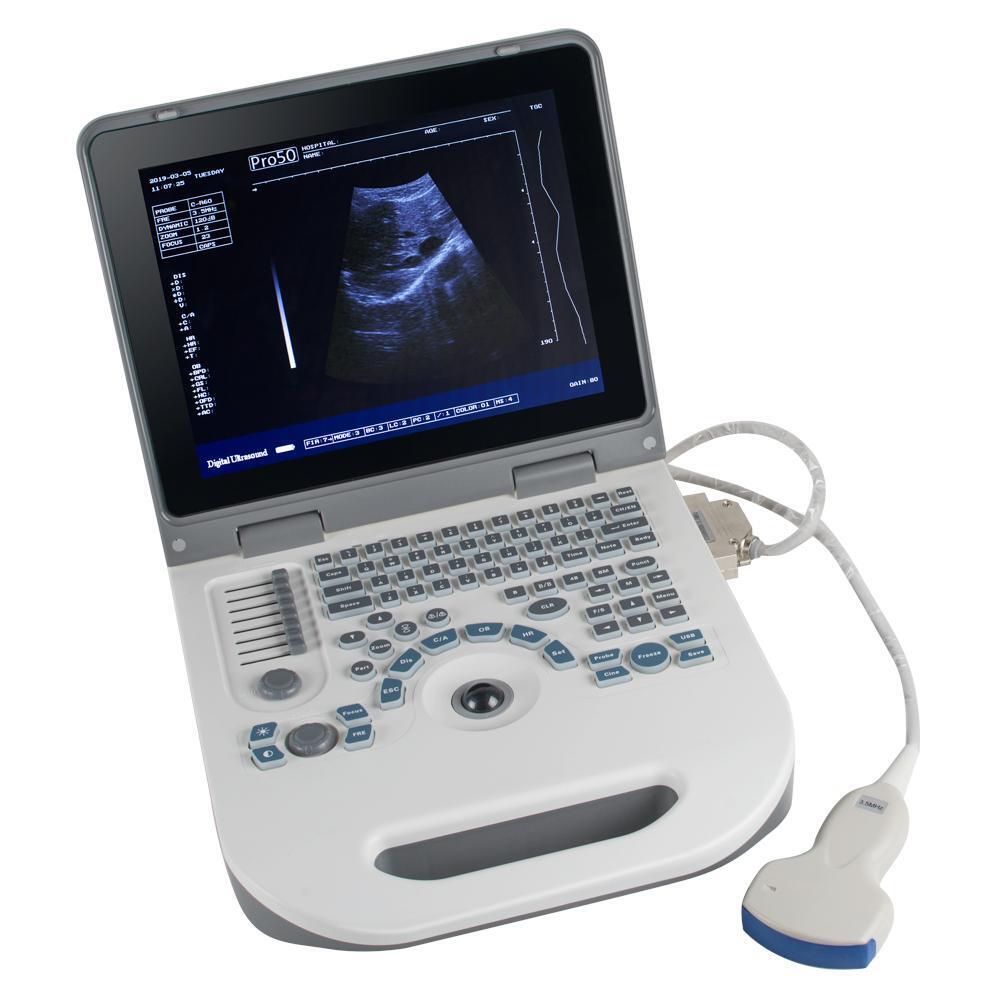
Handheld Ultrasound Scanner HandScanDescription:
Specifications:
Display Modes: B,B+B,B+M,M,4B
Frequency: 2.5MHZ-7.5MHZ
Scanning Depth: 70mm~220mm
Gray Scales: 256levels
Cine loop: 400frames
Image Storage: Built-in memory-64 images USB disk storage
Image Reservation: up/down,left/right
Image Conversion: distance,circumference,area,volume,EF rate,heart
Measurements: rate,Time
OB Measurements: BPD,GS,CRL,FL,HC,AC,EDD,GA,FWVIDEO (PLAD,NTSC),USB2.0
Monitor: 5.5 inch TFT color LCD
body markers: A variety of body markers(40 sorts)
Standard Configuration:
One(1)Main unit
One(1)R40/3.5mhz Convex Probe
One(1)AC-Adaptor
One(1)Rubber Protector
One(1)Rechargeable Battery
Two(2)shoulder and waist belts
ALL above will be packed in a carrying case
Comments: date&time,name,sex,age,hospital
Applications:Professional sofeware package in Obstetrics,Gynecology,Urology and Small parts
Weight:700g
Dimensions: 230mm×120mm×42mm
Battery working:3 hours
Optional:
Probes: R40/5.0mhz Micro-convex Probe
L40/7.5Mhz HF linear Probe
R10/6.5MHz Transvaginal Probe
Biopsy
Car Lighter
Additional rechargeable battery
FDA declaration :
Statement:The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item unless you are an authorized purchaser. If the item is subject to FDA regulation, I will verify your status as an authorized purchaser of this item before shipping of the item.
This item has been cleaned and treated according to the manufacturer's instructions.
The Fingertip Pulse Oximeter is certified with the US FDA 510K No. K070371, the CE & TUV of Eureope and it is on the Australian Register of Therapeutic Goods(ARTG) with the code 136606. div >
The Powered Surgical Instrument / Speed 808 System is certified with the US FDA 510(k) Number:K132989
The Powered Surgical Instrument / Hair Remove Device is certified with the US FDA 510(k) Number:K180353
The Powered Surgical Instrument / Hair Remove System is certified with the US FDA 510(k) Number:K141973
massager, vacuum, light induced heating / Slimming Treatment Device is certified with the US FDA 510(k) Number:K161892