-40%
Color Doppler Ultrasound Scanner Portable Laptop Machine Color Diagnostic Convex
$ 2428.27
- Description
- Size Guide
Description
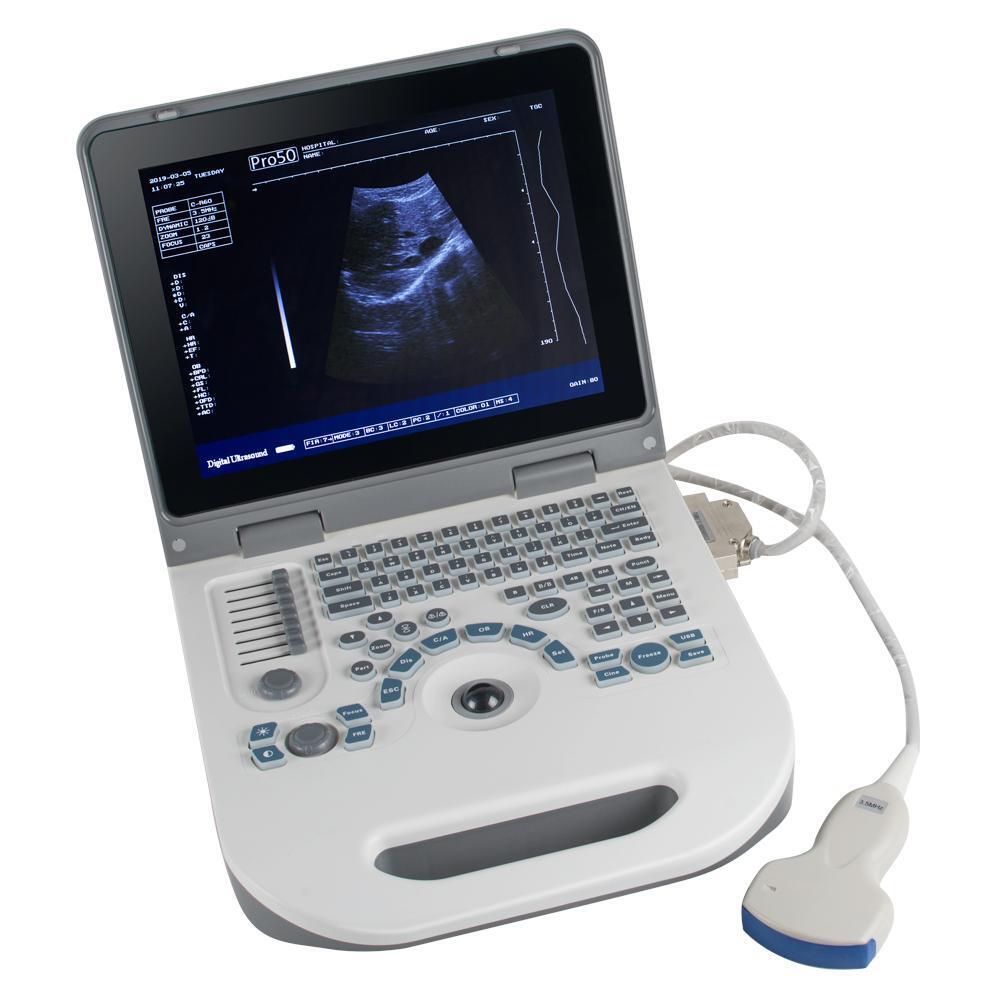
IntroductionCMS1700A is a color Doppler ultrasonic diagnostic device with high resolution, which has a powerful computer processing platform. The system is mainly suitable for the diagnosis of abdomen, heart, peripheral vessels, breast, obstetrics and gynecology, small organs, urology, muscle, incretion and pediatrics, etc. It adopts Doppler ultrasound imaging technology, advanced image processing technology (such as digital beam-forming technology, tissue harmonic imaging (THI), image speckle suppression, etc.) and digital integrated graphic management system, and the internal professional measurement software package can fully meet the clinic diagnostic requirement.
Features
1)Color Doppler (CF), color power Doppler imaging (PDI).
2)Pulse spectrum Doppler (PW).
3)B+CF (Dual Images)
4)B+CF/PDI/DPDI+PW (Triplex)
5)Linear array deflection/trapezoid imaging.(optional)
6)Anatomic M-mode imaging.(optional)
7)space compounding imaging
8)Tissue harmonic imaging (THI).
9)Wide scene imaging.(optional)
10)Puncture enhanced.(optional)
Performance
1)Display depth: ≥ 300 mm
2)Extended interface: VIDEO interface, S-VIDEO interface, RJ-45 interface, USB interface, VGA interface.
Safety
Type of protection against electric shock: class Ⅰ equipment
Degree of protection against electric shock: type B applied part
Operating voltage: AC 100 V~240 V
Operating frequency: 50 Hz/60 Hz
Power consumption: ≤ 100 VA
Accessories
One abdominal probe (3.5 MHz)
One User Manual
One power cord
One adapter
Physical characteristic
Dimension: 370 mm (L) × 360 mm (W) × 80 mm (H)
Weight: 6.5 kg
Payment
We accept Paypal Payment.
Shipping
Clearance: we will ship your item to your confirmed address.
Buyers' responsibility to pay duties,taxes and other extra charges by the government in your country.
Terms of Sale
Buy safe Products
The following FDA Disclaimer is required for all eBay listing in Healthcare category and is included for REFERENCE: The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies.If the item is subject to FDA regulation, We will verify your status as an authorized purchaser of this item before shipping of the item. If you have questions about legal obligations regarding sales of medical devices, you should consult with the FDA's Center for Devices and Radiological Health.
The Fingertip Pulse Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG) with the code 197923, and certified by FDA of United States and CE,TUV of Europe.The Fingertip Pulse Oximeter that is FDA 510K Approved.
About Us
Contec Medical Systems Co.,Ltd; 20 Years manufacturer,we have stock in USA and China.
Contact Us
If you have any questions for items,please do not hesitate to contact us through eBay Message, we are on line to reply your message within 24 hours.
Whatsapp:+86 17749297380; qiuyue0913(a)outlook.com