-40%
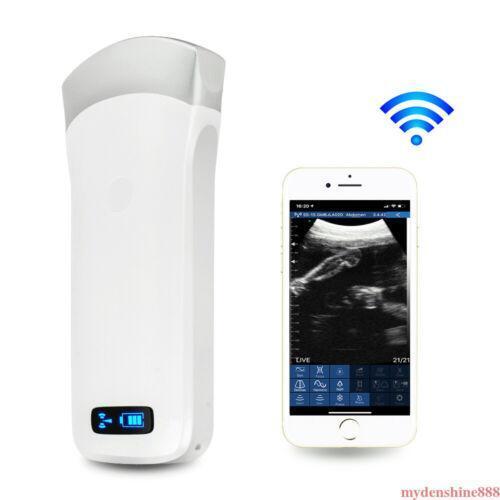
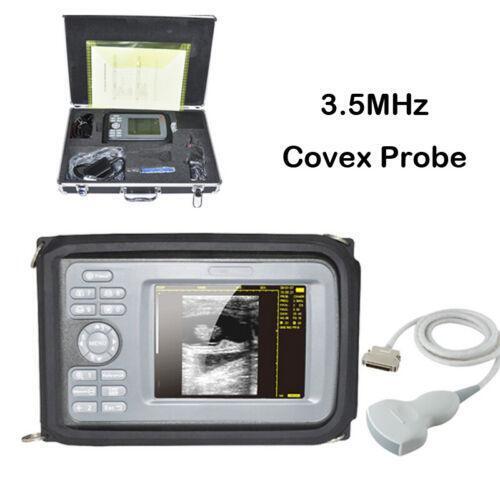
16 Channel Convex Probe 80 Elements Carejoy 3.5Mhz Wireless Ultrasound Machines
$ 639.82
- Description
- Size Guide
Description
High cost-effectiveAdvanced digital imaging technology, clear image
Intelligent terminal platform, powerful expansion functions on application, storage, communication,printing
Small and light, easy to carry and operate
Description:
Specifications:
Scanning mode: Electronic array
Display mode: B, B/M
Probe frequency: 3.5MHz
Scan depth: 90/160/220/305mm
Scan angle: 60°
Head radius: 60mm
Image Adjust: BGain,Focus, Depth, Harmonic, noise reduction
Cineplay: auto and manual, frames can set as 100/200/500/1000
Puncture assist function: the function of inplane puncture guide line, out-of
plane puncture guide line, automatic blood vessel measurement.
Measure: Length, Area, Angle, Heart rate, Obstetrics
Image save: jpg, avi and DICOM format
Image frame rate: 22 frames/second
Dimension: 11*6*2cm
Weight: 120g
Working system:Apple iOS and Android, Windows
Standard Configuration:
1×Probe Type Ultrasound Scanner
1×USB Connect and Charging Cable
FDA declaration :
Statement:The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item unless you are an authorized purchaser. If the item is subject to FDA regulation, I will verify your status as an authorized purchaser of this item before shipping of the item.
This item has been cleaned and treated according to the manufacturer's instructions.
The Fingertip Pulse Oximeter is certified with the US FDA 510K No. K070371, the CE & TUV of Eureope and it is on the Australian Register of Therapeutic Goods(ARTG) with the code 136606. div >
The Powered Surgical Instrument / Speed 808 System is certified with the US FDA 510(k) Number:K132989
The Powered Surgical Instrument / Hair Remove Device is certified with the US FDA 510(k) Number:K180353
The Powered Surgical Instrument / Hair Remove System is certified with the US FDA 510(k) Number:K141973
massager, vacuum, light induced heating / Slimming Treatment Device is certified with the US FDA 510(k) Number:K161892
On Dec 13, 2022 at 08:15:22 PST, seller added the following information: